Edwards SAPIEN 3 Pulmonic Portfolio:
Unique Patients
Excellent Outcomes
Edwards SAPIEN 3 Pulmonic Portfolio:
Unique Patients
Excellent Outcomes
Dedicated to your pulmonic patients
Edwards Lifesciences is committed to giving more patients less invasive procedures.
Your pulmonic patients face a lifetime of invasive surgical procedures, and you are looking for therapy options to lessen the burden.
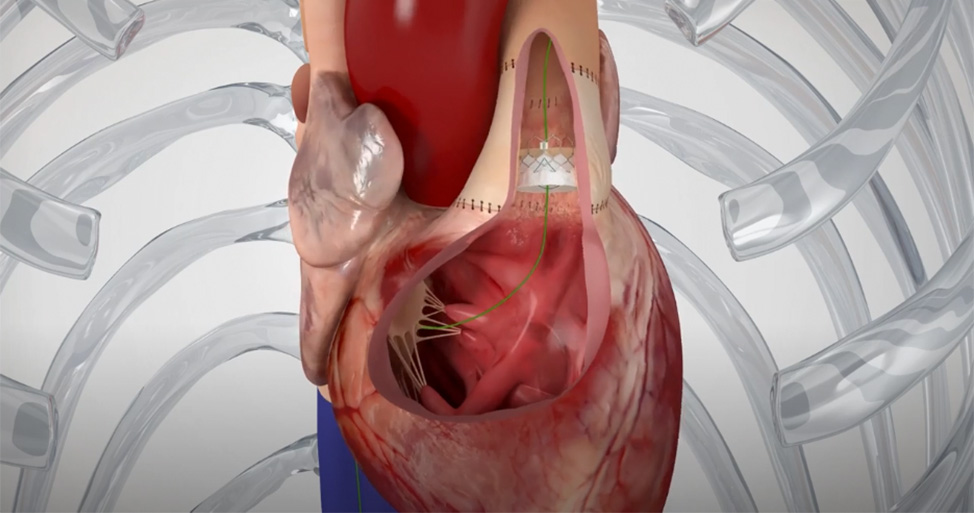
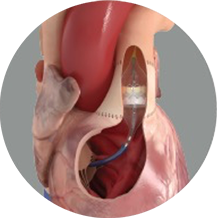
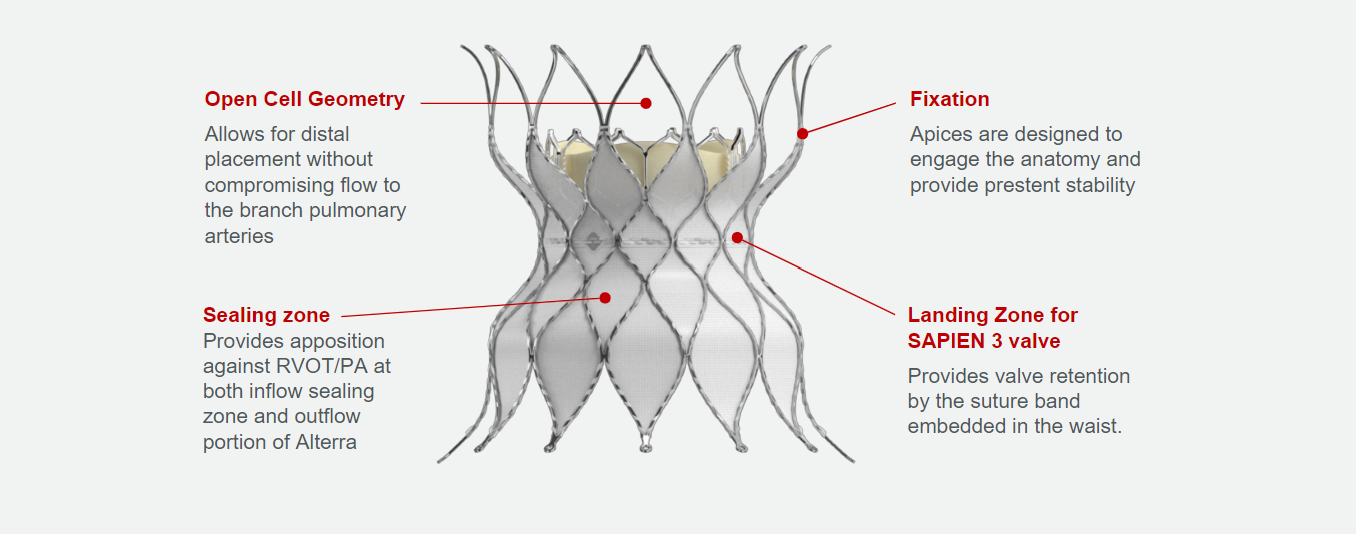
That's why the Edwards Alterra adaptive prestent was developed. It allows the SAPIEN 3 valve to reach a broader range of pulmonic patients and delays their potential need for additional open-heart surgery.1
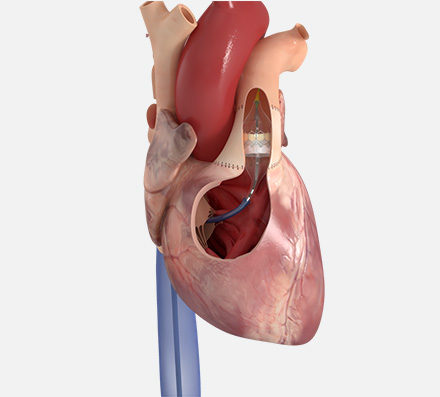
Continue putting your pulmonic patients first with the SAPIEN 3 valve
A less invasive alternative to surgery that can adapt to your patients' needs
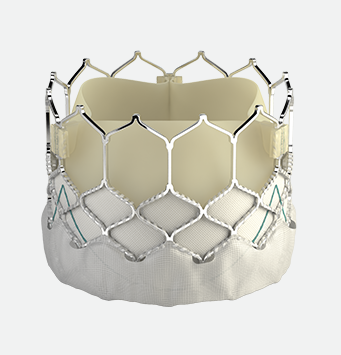
The SAPIEN 3 transcatheter pulmonic valve
Available in 20, 23, 26, and 29 mm
The Edwards SAPIEN 3 transcatheter heart valve system is indicated for use in patients with a dysfunctional, previously repaired or replaced non-compliant Right Ventricular Outflow Tract/Pulmonary Valve (RVOT/PV) or previously implanted valve in the pulmonic position.*
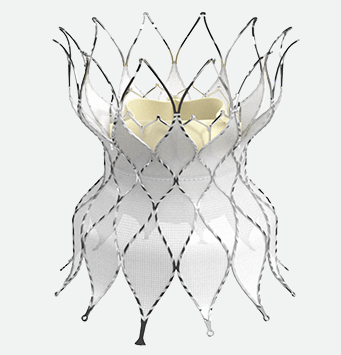
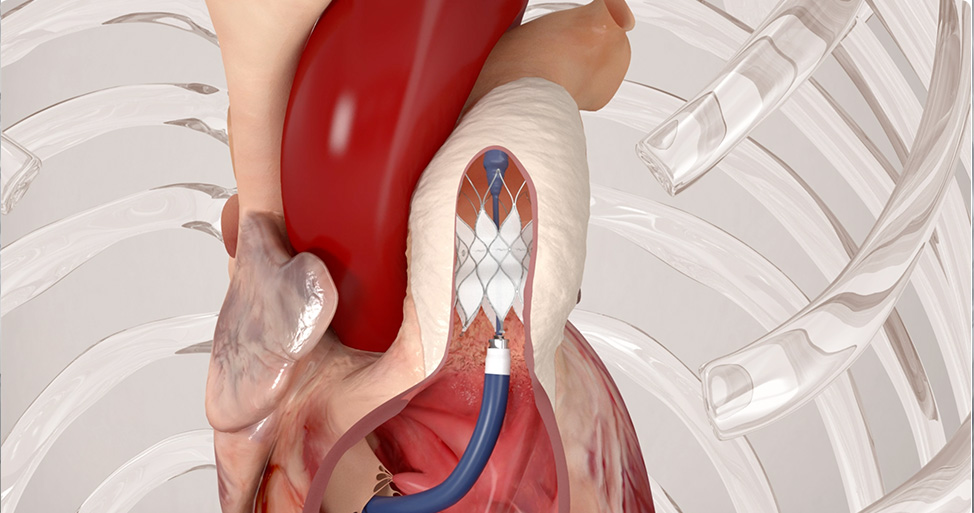
The SAPIEN 3 transcatheter pulmonic valve with Alterra adaptive prestent
Adapts to the anatomy for placement of a 29 mm SAPIEN 3 valve2, 3
The Edwards SAPIEN 3 transcatheter pulmonary valve system with Alterra adaptive prestent is indicated for use in the management of pediatric and adult patients with severe pulmonary regurgitation as measured by echocardiography who have a native or surgically-repaired right ventricular outflow tract and are clinically indicated for pulmonary valve replacement.
A proven transcatheter technology, now available for your pulmonic patients5
Delays the potential need for future surgical intervention with a less invasive alternative for patients with a history of pulmonary valve intervention4
Excellent Clinical Performance4
0%
All cause death at 1 year* (n=51)
Endocarditis at 1 year* (n=51)
Valve frame fracture at 1 year* (n=55)
100%
Freedom from surgical reintervention at 1 year* (n=56)
98.1%
Device success rate*† (n=56)
97.9%
≤ Moderate/severe paravalvular regurgitation at 1 year (n=47)
The major risks associated with the transcatheter pulmonic valve procedure include death, heart damage potentially requiring surgery, bleeding, blood vessel complications, and irregular heart beat.
*COMPASSION S3 trial results using the 20, 23, 26, and 29 mm SAPIEN 3 valve, valve implant population (n=56)
†Device success is a composite of
- Single THV implanted in the desired location
- RV-PA peak-to-peak gradient < 35 mmHg post implantation
- Less than moderate PR by discharge TTE (or earliest evaluable TTE)
- Free of explant at 24 hours post implantation
Edwards Commander Delivery System for SAPIEN 3 pulmonic valve implantation
Edwards Commander delivery system brings predictability and control to your pulmonic procedures as well as dual articulation for coaxiality

- Allows valve positioning and deployment
- Dual articulation for coaxiality
- Accurate positioning and deployment
- Compatible with 14F /16F eSheath+ introducer set
- Compatible with 24F Gore DrySeal sheath (20, 23, and 26 mm valves) or 26F sheath (29 mm valve)
A less invasive option for more pulmonic patients
Alterra adaptive prestent bridges the gap when pulmonic patients need it most
Delivering excellent clinical outcomes with sustained performance at 2 years6
Excellent outcomes*
- 0% mortality
- 0% endocarditis
- 0% stent fracture requiring reintervention
Sustained performance
- 1.7% reintervention*
- 92.5% ≤ mild pulmonary regurgitation**
*n=59
**n=53
Precise predictable placement
- 100% single Alterra prestent deployed in
desired location - 96.7% SAPIEN 3 valve deployed in
desired location
Trackablity where you want it, recapturability when you need it
With two distinct delivery systems you can focus on optimizing outcomes by further evaluating positioning before valve implantation. Additionally, recapturability of the Alterra adaptive prestent allows you to reposition as needed for precise placement.
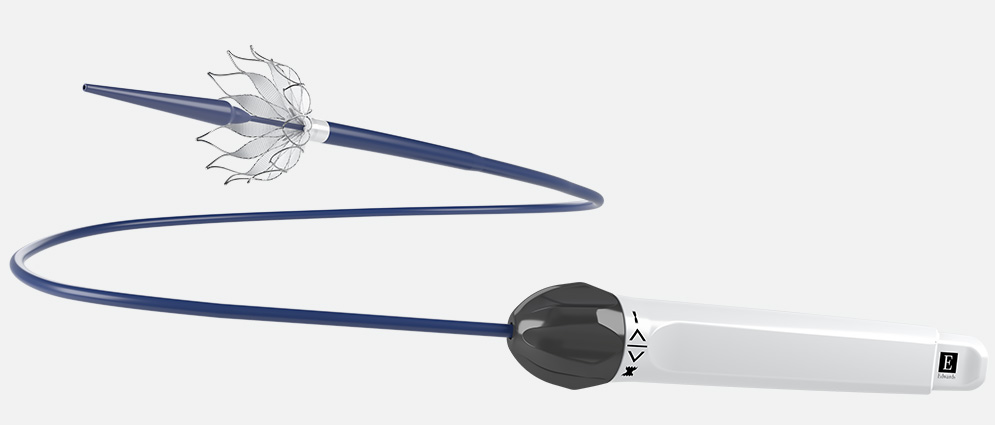
Alterra adaptive prestent delivery system
- Affords the option to recapture and reposition, as needed, for accurate placement
- Ergonomic design for controlled deployment
- Designed for smooth tracking to the pulmonic artery
- Compatible with the 16F eSheath+ introducer set
- Preloaded prestent
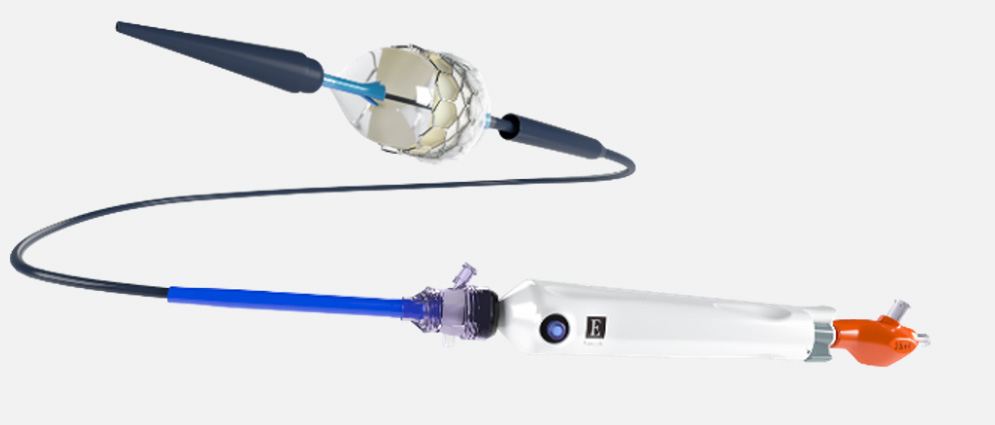
SAPIEN 3 pulmonic delivery system
- Facilitates SAPIEN 3 valve delivery into Alterra adaptive prestent
- Ability to track to the intended location through vasculature
Explicitly designed for pulmonic therapy, the Edwards SAPIEN 3 transcatheter pulmonary valve system with Alterra adaptive prestent allows you to keep making an impact in the lives of your patients
Reach a broader range of pulmonic patients with the Edwards SAPIEN 3 valve and Alterra adaptive prestent
References:
1. FDA Summary of Safety and effectiveness data, December 2021
2. Edwards Lifesciences data on file
3. SAPIEN 3 with Alterra adaptive prestent IFU
4. Lim S, et al. Congenital Pulmonc Valve Dysfunction Treated with SAPIEN 3 Transcatheter Heart Valve (from the COMPASSION S3 Trial., Am J Cardiol. 2023;190:102-109.
5. Hascoët S, et al.Outcomes of transcatheter pulmonary SAPIEN 3 valve implantation: an international registry, Eur Heart J. 2024;45(3):198-210.
6. Shahanavaz, Shabana. Transcatheter Pulmonary Valve Implantation with the Alterra Adaptive Prestent and SAPIEN 3 Transcatheter Heart Valve: Two-Year Outcomes of the Alterra Pivotal Trial; Presented at PICS-AICS 2023, Washington DC.
* Edwards SAPIEN 3 Transcatheter heart valve IFU
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
PP--EU-9135 v1.0