TAVI value and optimisation
Uncover the hidden benefits of Edwards SAPIEN 3 TAVI heart valves
TAVI value and optimisation
Uncover the hidden benefits of Edwards SAPIEN 3 TAVI heart valves
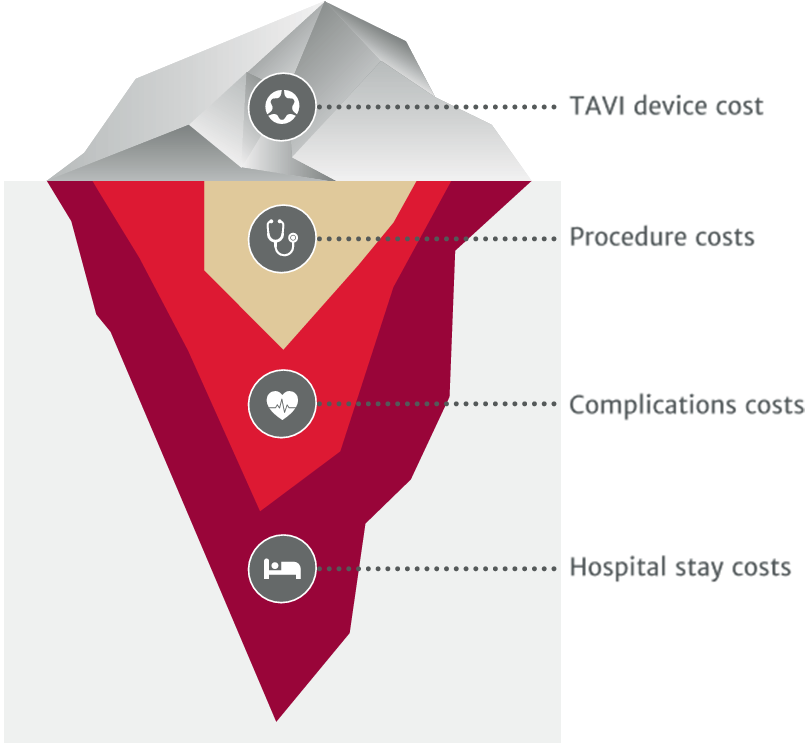
Value through excellent clinical outcomes
Edwards SAPIEN 3 valve and Edwards SAPIEN 3 Ultra valve have been shown to deliver high procedural success rates with consistently low-level complications.
Taking your TAVI outcomes to new heights:
- Low mortality and stroke1
- Minimal ≥ moderate paravalvular leak1,2
- Single digit permanent pacemaker rates.1-5

Learn more about how Edwards SAPIEN 3 TAVI clinical results translate into hospital health economic benefits:
Value through improved patient’s quality of life (QoL)
Among low-risk patients with severe aortic stenosis (sAS), TAVI with Edwards SAPIEN 3 valve was associated with meaningful early and late QoL benefits compared with surgery.6
Evaluating QoL with the Kansas City Cardiomyopathy Questionnaire (KCCQ)7
The KCCQ is a highly reliable, responsive, and valid measure of symptoms, functional status, and QoL in patients with severe, symptomatic aortic stenosis (sSAS).7
23 items scored across 5 domains of health status (Physical limitations; Social limitations; Symptoms; Quality of life; Self-efficacy) combine into a global summary scale (KCCQ Overall Summary).

Interpreting the change in KCCQ scores from baseline to post-procedure
Change in KCCQ Overall Summary Score7
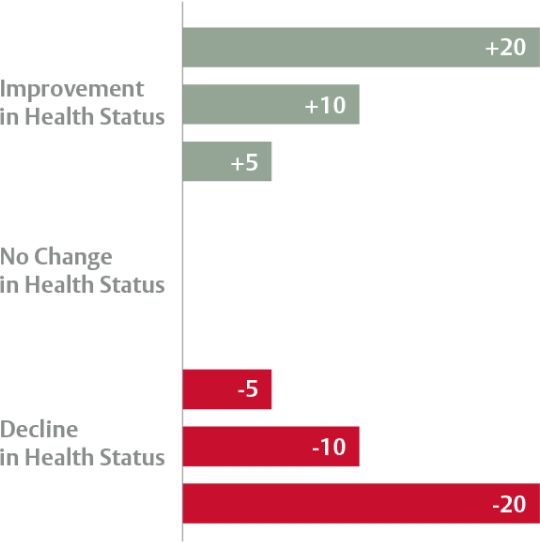
Edwards SAPIEN 3 TAVI provided consistently high early and lasting QoL benefits
Early (30 day) QoL benefit with Edwards SAPIEN 3 TAVI persisted at 6 months and 1 year in low risk patients.6
KCCQ-Overall Summary Score — differences from baseline

Interpreting the change in KCCQ scores from baseline to post-procedure
Edwards SAPIEN 3 TAVI provided consistently high early and lasting QoL benefits
Change in KCCQ Overall Summary Score7
Early (30 day) QoL benefit with Edwards SAPIEN 3 TAVI persisted at 6 months and 1 year in low risk patients.6
KCCQ-Overall Summary Score — differences from baseline


Organisational benefits
The increasing ageing population is driving the incidence of heart valve disease up.8,9
- Your hospital's ability to tackle this imminent health challenge will be critical
- Increasing demands on capacity for life-saving heart valve disease services can be managed efficiently with TAVI for sSAS

Transfemoral Edwards SAPIEN 3 TAVI offers:3,4
- Increased sAS patient turnover due to shortened procedural times allows you to treat more patients with the same resources
- Safe and quick patient recovery times
- A short hospital stay
- Reduced use of intensive care resources
Where indicated, a streamlined, less invasive solution for sSAS patients that frees up access and capacity to successfully manage and perform more heart valve procedures within limited resources.
The Edwards Benchmark program best practices support higher efficiencies with ambitious outcome targets
Learn about the Edwards Benchmark ProgramEconomic benefits and efficiencies
Why health economic evaluations are important:
With high level clinical and economic insights, we can strategically overcome financial barriers — helping to ensure TAVI procedures are rationally funded and accessible to all eligible patients.
Budget impact and cost-effectiveness

Measures the increased cost of the new technology vs current practice.

Establishes the new benchmark for the health benefits gained and the costs incurred when introducing a new technology.
Inform your economic decision-making regarding Edwards SAPIEN 3 TAVI vs. surgical aortic valve replacement (SAVR)
Edwards SAPIEN 3 TAVI vs SAVR has been found to dominate:
- Cost-effectiveness: better clinical outcomes at affordable cost.12
- Cost-saving: better clinical outcomes and cheaper in EU and USA.12,13
Cost effectiveness analysis and health economic assessment of TAVI vs SAVR
The economic outcomes of TAVI vs SAVR for low-risk patients
See results from PARTNER 3 TrialHow Edwards SAPIEN 3 valve and Edwards SAPIEN 3 Ultra valve may improve your TAVI program efficiency in your hospital
Making savings from admission to discharge

Short procedure time can increase hospital efficiency and turnover14

Low complication rate drives the cost of TAVI down15

Early discharge times reduce hospital TAVI costs significantly16

Edwards SAPIEN 3 Platform clinical efficacy and outcomes
Find out moreContact your Edwards Lifesciences sales representative for more information
Stay informed with our newsletter
Thank you!
Thank you for signing up to the heartvalves.com newsletter. You will now receive emails on the latest developments and industry insights on heart valve innovation and technology.
References:
1. Nazif T, Daniels D, McCabe J, Chehab B, et al. Real-world experience with the SAPIEN 3 Ultra TAVI: A propensity matched analysis from the United States. Presented virtually at TVT Connect 2020.
2. Saia F, Gandolfo C, Palmerini T, et al. In-hospital and thirty-day outcomes of the SAPIEN 3 Ultra balloon-expandable TAVR: the S3U registry. Eurointervention 2020;15(14):1240-1247.
3. Wood, DA, Lauck SB, Cairns JA, et al. The Vancouver 3M (multidisciplinary, multimodality, but minimalist) clinical pathway facilitates safe next-day discharge home at low-, medium-, and high-volume transfemoral transcatheter aortic valve replacement centers: the 3M TAVI study. J Am Coll Cardiol Intv. 2019;12(5):459–469.
4. Barbanti M, van Mourik MS, Spence MS, et al. Optimising patient discharge management after transfemoral transcatheter aortic valve implantation: the multicentre European FAST-TAVI trial. EuroIntervention. 2019;15:147–154.
5. Yamamoto M, Watanabe Y, Tada N, et al. Transcatheter aortic valve replacement outcomes in Japan: optimized catheter valvular intervention (OCEAN) Japanese multicenter registry. Cardiovasc Revasc Med. 2019;20(10):843–851.
6. Baron SJ, Magnussen AA, Lu M, et al., Health Status after Transcatheter vs. Surgical Aortic Valve Replacement in Low-Risk Patients with Aortic Stenosis. JACC 2019;74(23):2833–2842.
7. Arnold SV, Spertus JA, Lei Y, et al. Use of the Kansas City Cardiomyopathy Questionnaire for Monitoring Health Status in Patients with Aortic Stenosis. Circulation Heart Failure. 2013;6:61–67
8. d’Arcy J, Coffey S, Loudon MA, et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the OxVALVE Population Cohort Study. Eur Heart J. 2016 Dec 14;37(47):3515–3522. Available at: https://www.ncbi.nlm.nih.gov/pubmed/27354049
9. European Society of Cardiology. Epidemiology of aortic valve stenosis (AS) and of aortic valve incompetence (AI): is the prevalence of AS/AI similar in different parts of the world? Available at: https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-18/epidemiology-of-aortic-valve-stenosis-as-and-of-aortic-valve-incompetence-ai
10. Leelahavarong P. Budget impact analysis. J. Med Assoc Thai 2014;97(Suppl 5):S65–S71 (Abstract).
11. WHO. Cost-effectiveness analysis for health interventions. https://www.who.int/heli/economics/costeffanalysis/en/
12. Butala N. Economics of minimalist TAVR: The 3M TAVR Economic Study. Presented at: TVT 2021. July 21, 2021.
13. Gilard M, Eltchaninoff H, Iung B, et al. Cost-Effectiveness Analysis of SAPIEN 3 Transcatheter Aortic Valve Implantation Procedure Compared With Surgery in Patients With Severe Aortic Stenosis at Low Risk of Surgical Mortality in France. Value in Health. 2021;(Article in press). Accessed online 25.11.2021
14. Wayangankar SA, Elgendy IY, Xiang Q, et al. Length of Stay After Transfemoral Transcatheter Aortic Valve Replacement: An Analysis of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry, JACC Cardiovasc Interv 2019;12(5):422–430.
15. Gutmann A, Kaier K, Sorg S, et al, Analysis of the Additional Costs of Clinical Complications in Patients Undergoing Transcatheter Aortic Valve Replacement in the German Health Care System. Int J Cardiol.2015;179:231–237.
16. Chevreul K, Brunn M, Cadier B, et al. Cost of transcatheter aortic valve implantation and factors associated with higher hospital stay cost in patients of the FRANCE (FRench Aortic National CoreValve and Edwards) registry. Arch Cardiovasc Dis. 2013;106(4):209–219.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
PP--EU-3366 v1.0