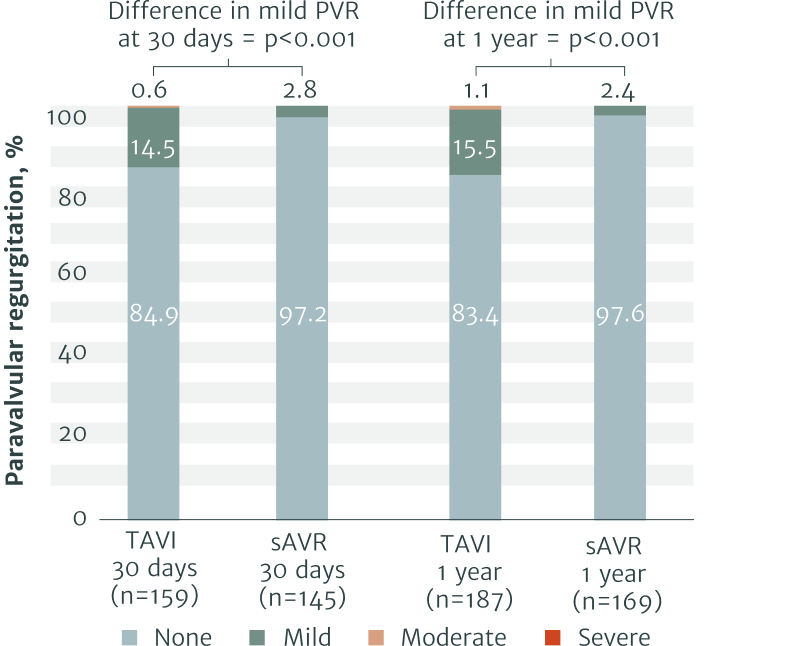
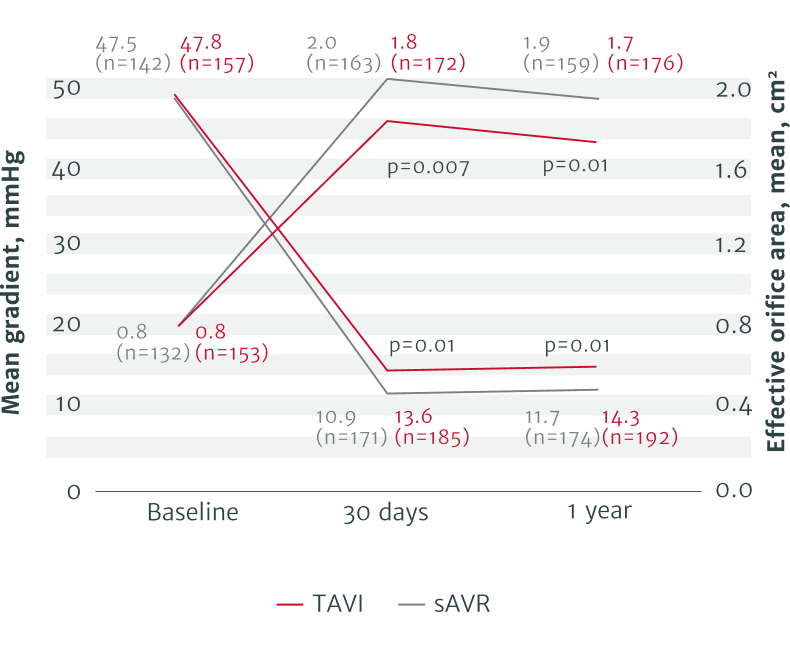
The RHEIA Trial: Female-only, investigator-initiated study
Randomized researcH in womEn all comers wIth Aortic stenosis
Eltchaninoff H et al. RHEIA – Transcatheter versus surgical aortic valve replacement in women with severe aortic stenosis. Presented at ESC Congress, 30 August–2 September 2024, London, UK.