2021 ESC/EACTS VHD Guidelines
A new treatment pathway for severe aortic stenosis (sAS) patients
2021 ESC/EACTS VHD Guidelines
A new treatment pathway for severe aortic stenosis (sAS) patients
Treatment and guidelines
- All severe aortic stenosis (sAS) patients* should now be referred to the Heart Team for a treatment evaluation1
- The guidelines recommend transfermoral transcatheter aortic valve implantation (TF-TAVI) as preferred standard of care (class IA recommendation) in patients ≥75 years of age**, as well as for additional patient groups <75 years of age1
- The Heart Team recommendation should be discussed with the patient, who can then make an informed treatment decision between the option of TAVI or surgical aortic valve replacement (SAVR)1
Aortic Valve Replacement (AVR) treatment modality is now based on age and not surgical risk as first deciding factor1
The evidence supporting these changes originated from multiple real-world studies, registries and randomized controlled trials.1,2
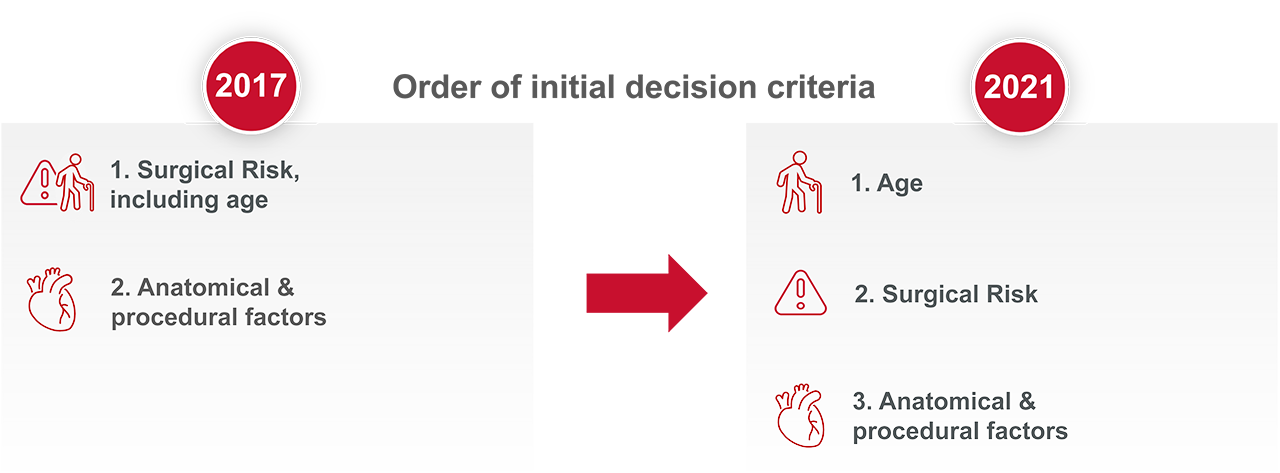
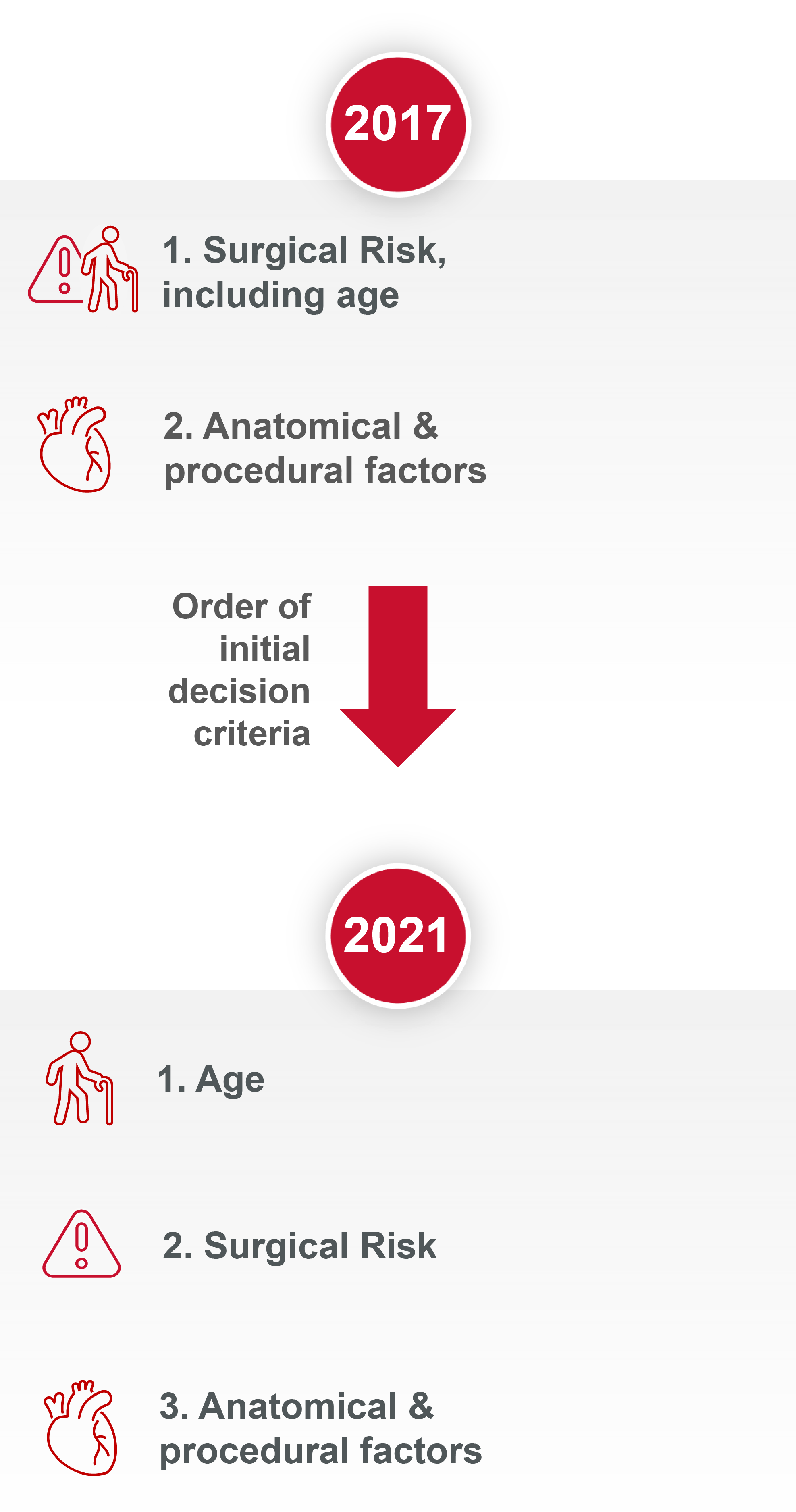
How the evidence has modified the aortic stenosis (AS) treatment recommendations
Since the publication of the 2017 guidelines, new evidence has accumulated. This has led to a modification of both the AS treatment recommendations and the strength of those recommendations within the new 2021 publication.
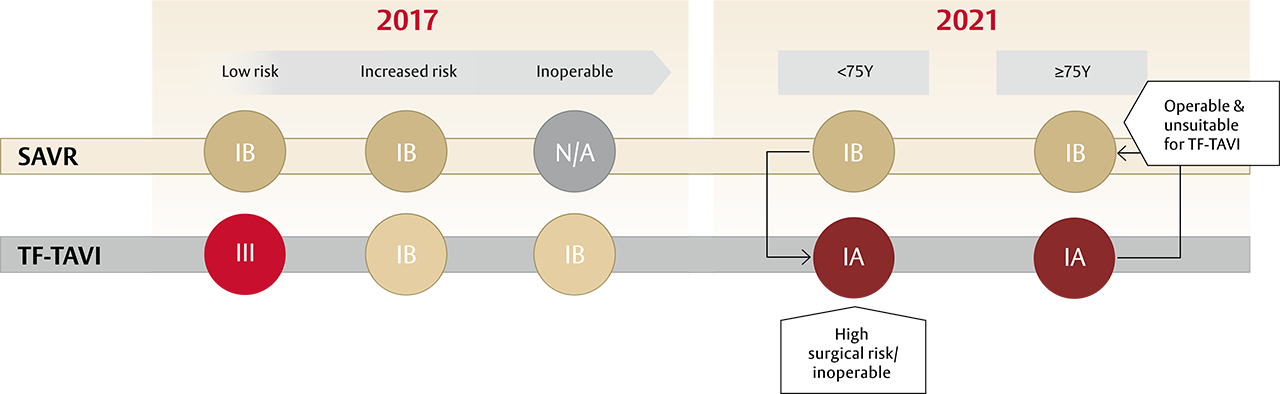
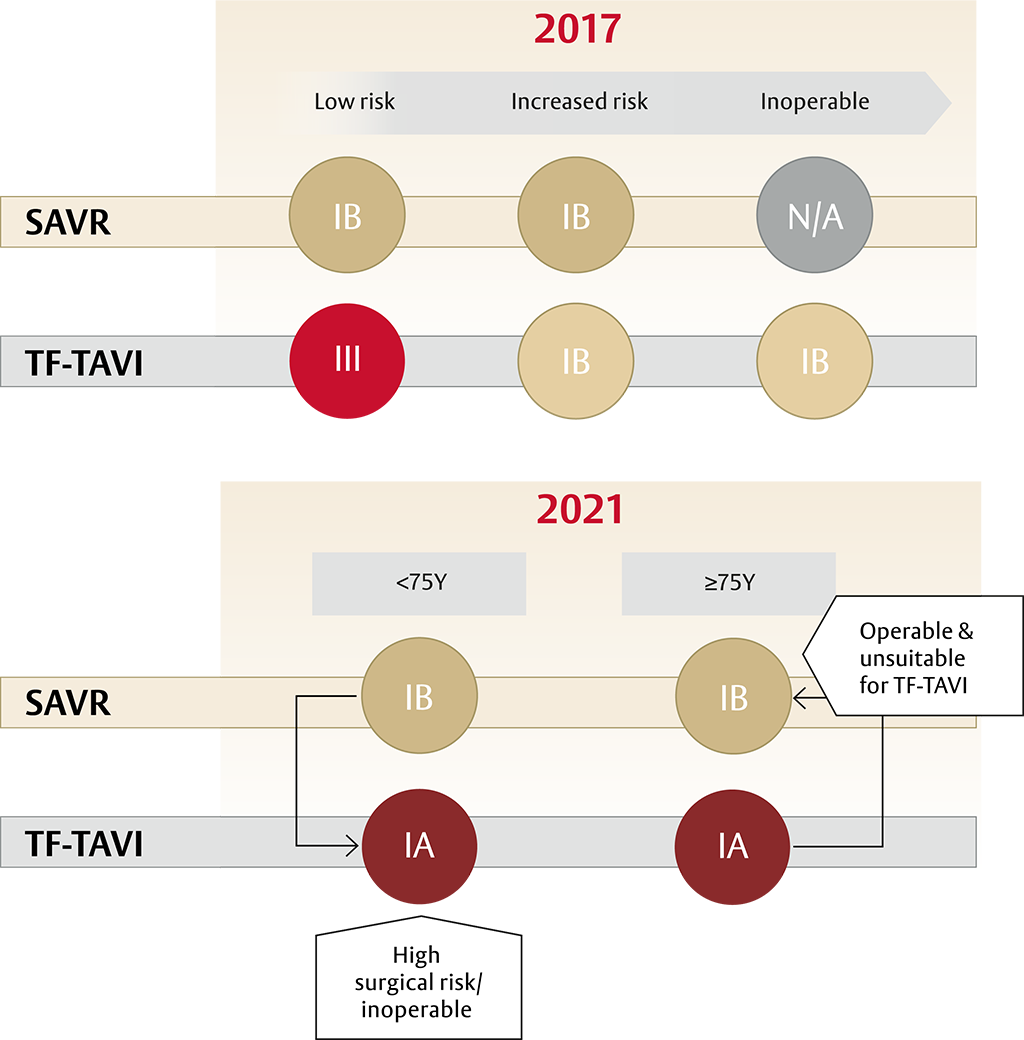
A new standard of care for all patients with sAS* ≥75 years of age: TF-TAVI1
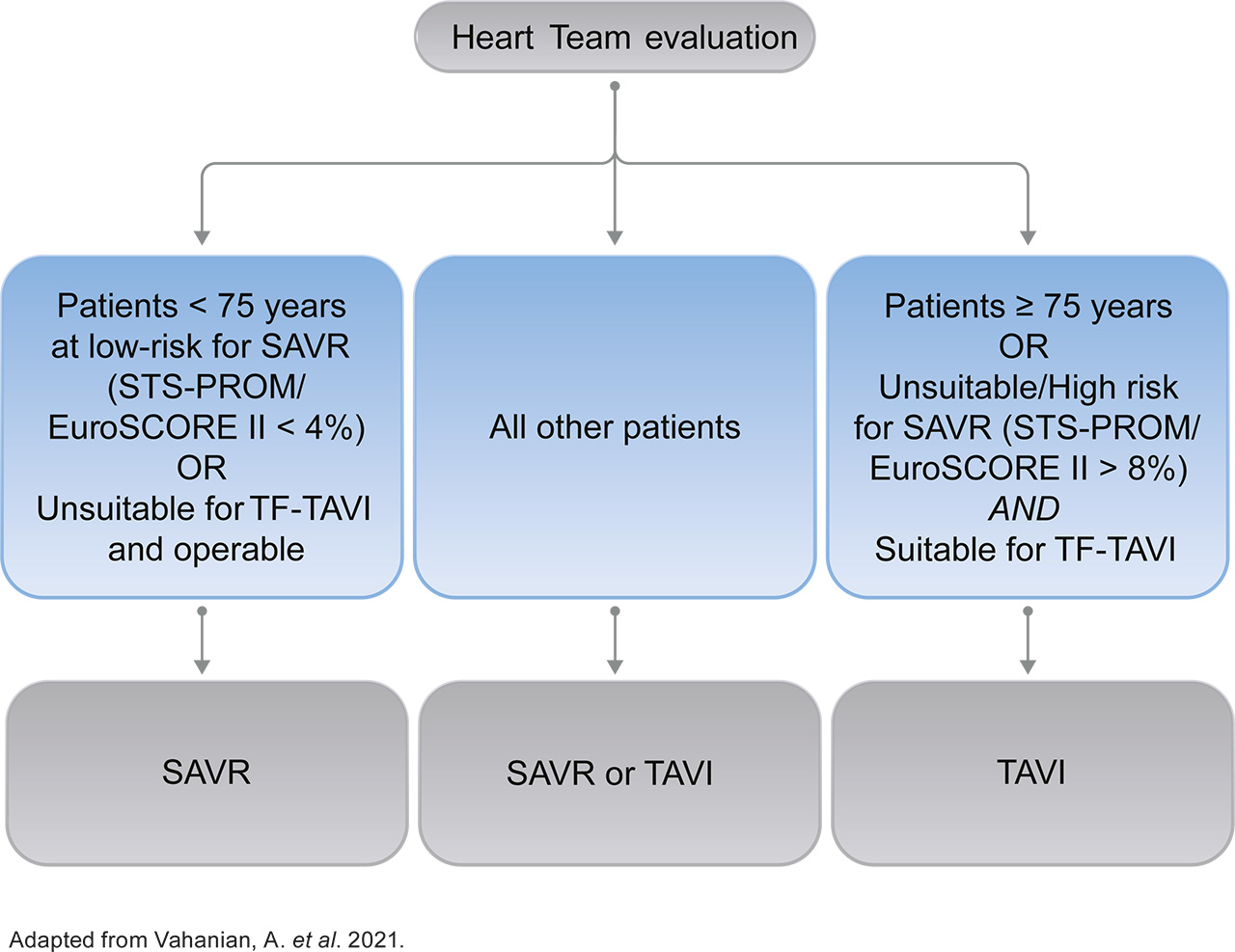
After patients have been referred on age — surgical risk, anatomical and procedural factors, comorbidities and frailty need to be assessed1
TAVI may be favourable in:
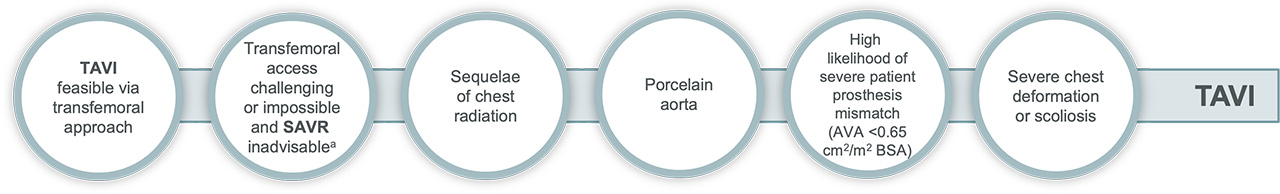
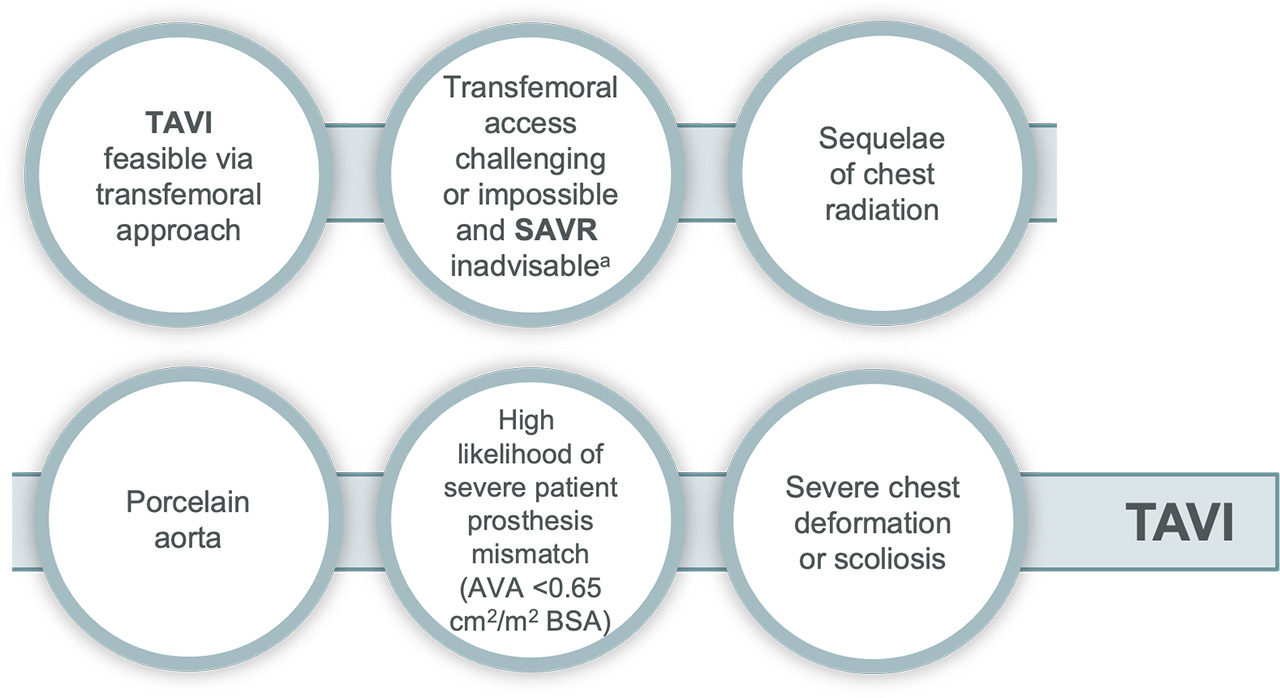
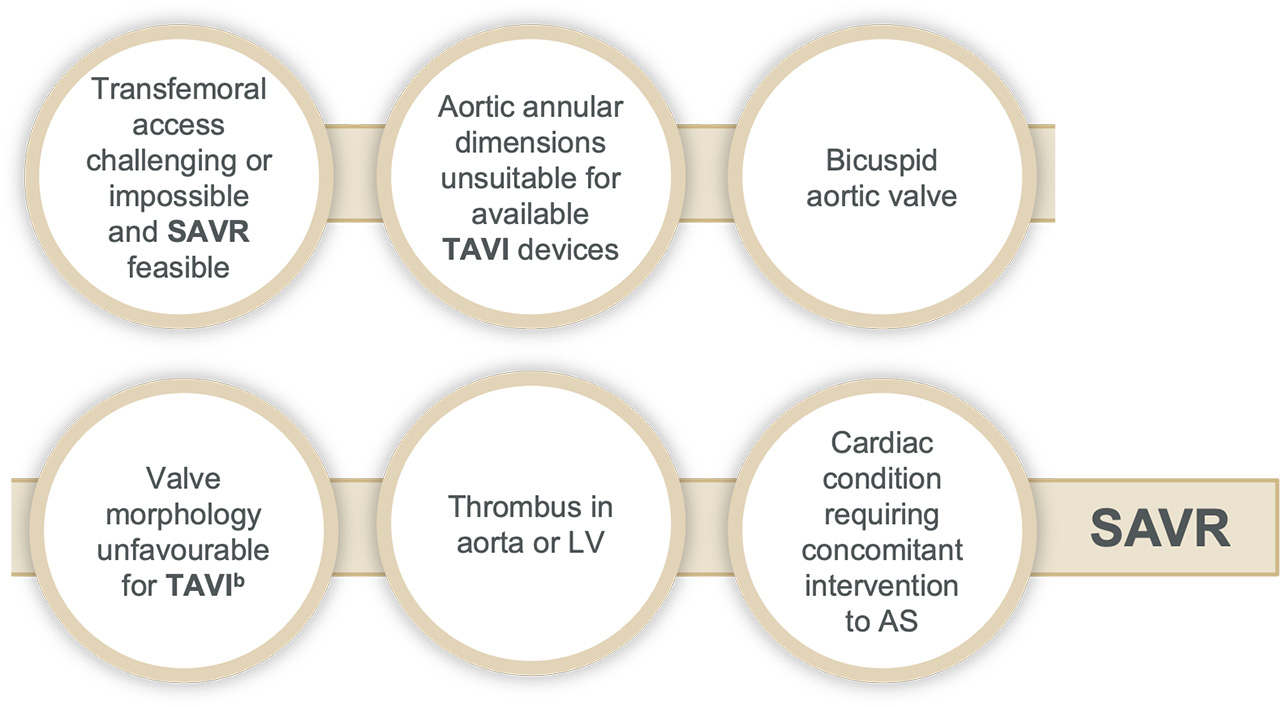
Surgery may be favourable in:
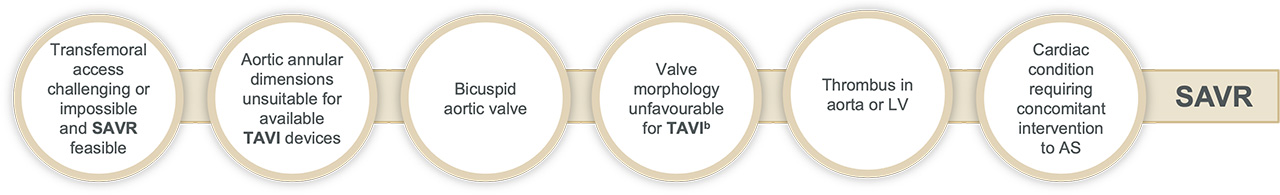
The guidelines recommend that all patients with sAS* are evaluated by the Heart Team and included in treatment decisions
The 2021 ESC/EACTS guidelines on AS treatment emphasise the need for patients to understand and decide on their preferred treatment.

Stay informed with our newsletter
Thank you!
Thank you for signing up to the heartvalves.com newsletter. You will now receive emails on the latest developments and industry insights on heart valve innovation and technology.
*With a treatment indication
**Based on evaluation of clinical, anatomical and procedural factors
aVia non-transfemoral approach
bFor example: high risk of coronary obstruction due to low coronary ostia or heavy leaflet/LVOT calcification
References:
1. Vahanian A, Beyersdorf F, Praz F, et al. Eur Heart J. 2021; ehab395. doi:10.1093/eurheartj/ehab395
2. Baumgartner H, Falk V, Bax JJ, et al. Eur Heart J. 2017; ehx391. doi:10.1093/eurheartj/ehx391
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
PP--EU-3365 v1.0