掌握未來新趨勢
為了滿足病患的需求,保持在學術上精益求精將使我們達到更高的理想
掌握未來新趨勢
為了滿足病患的需求,保持在學術上精益求精將使我們達到更高的理想
術後維持病患的良好生活品質是您遠大的目標。利用SAPIEN 3 Ultra 進行經導管主動脈瓣置換術將大大滿足主動脈瓣狹窄患者的需求。
點擊以閱讀更多
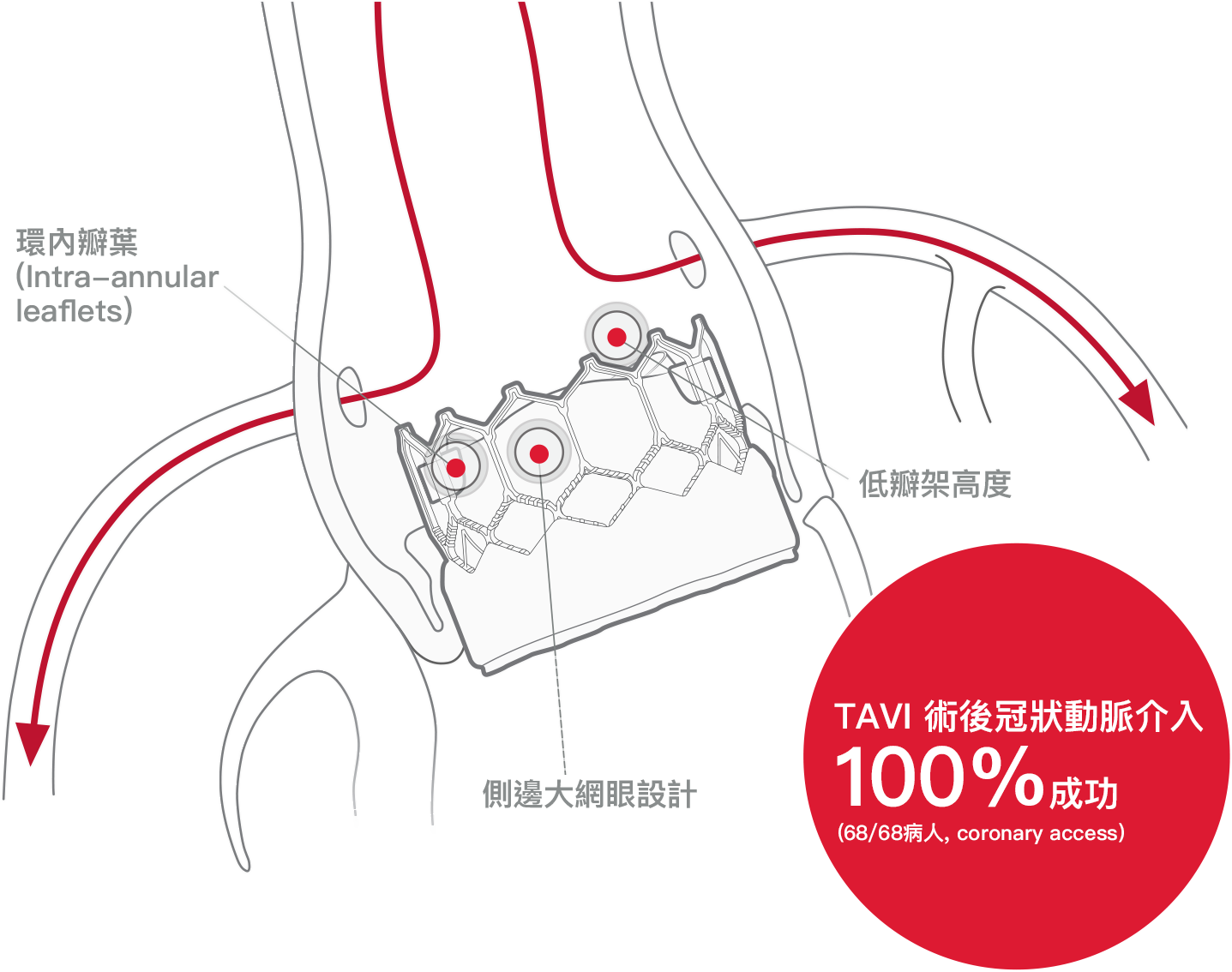
*研究僅包含使用SAPIEN 3瓣膜
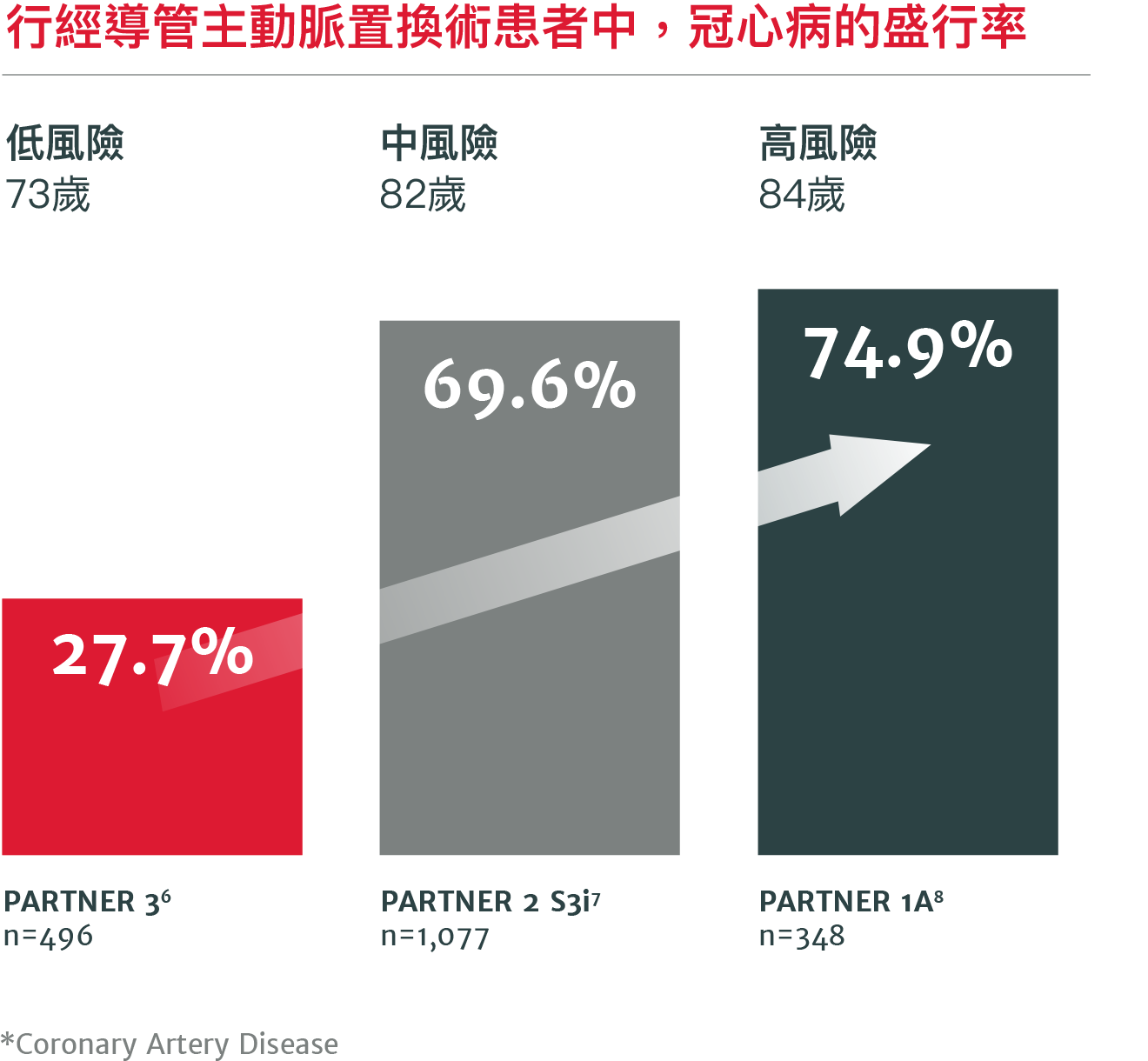
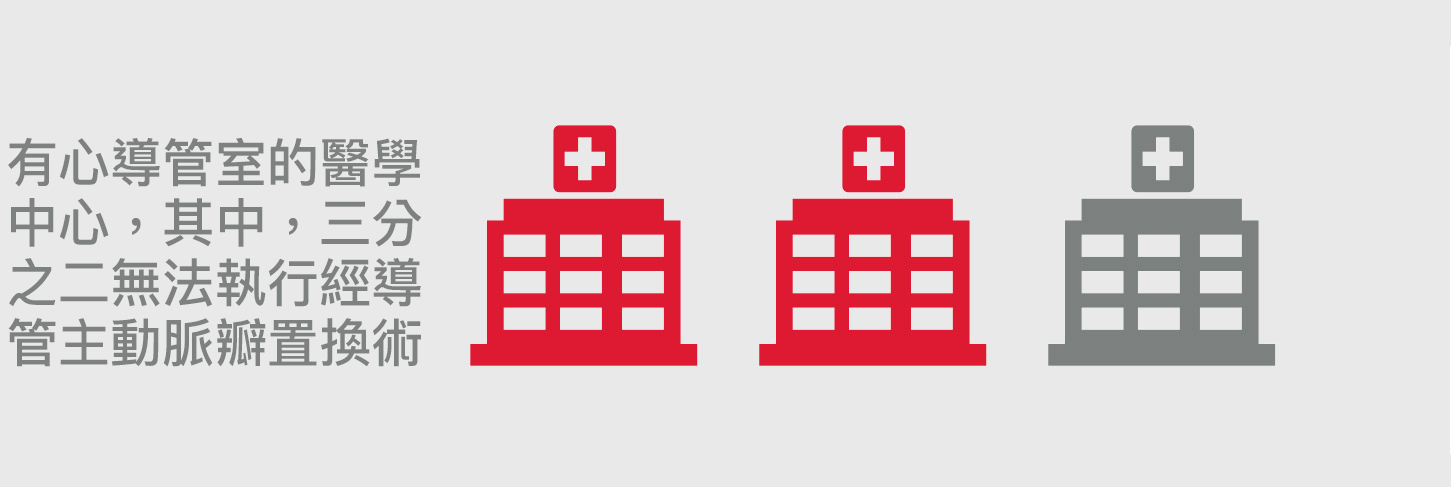
高達70%行經導管主動脈瓣置換術的病患將來會因冠心病而需再進行處置5
術後若需要進行冠狀動脈相關手術可以由對經導管主動脈瓣置換術經驗相對較少的醫師來進行3
經主動脈瓣膜置換術的瓣膜耐用性在術後五年內和外科主動脈瓣膜置換術(SAVR)不相上下
在PARTNER II試驗中,術後五年發生瓣膜的結構性損壞4
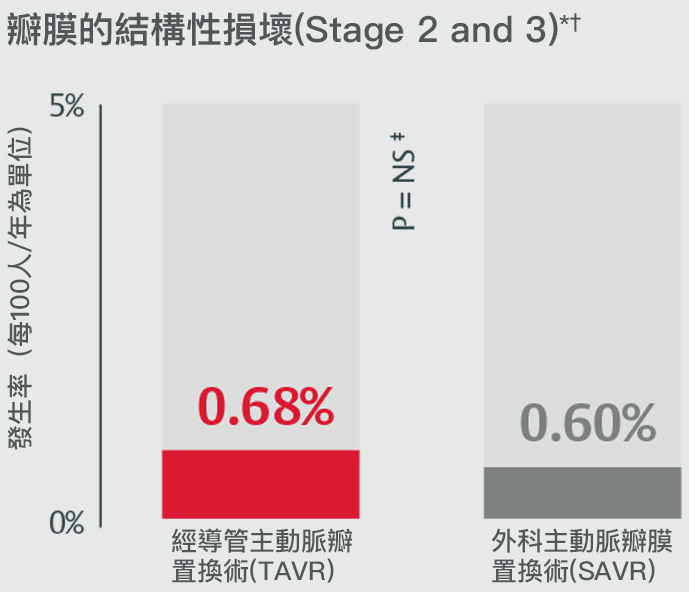
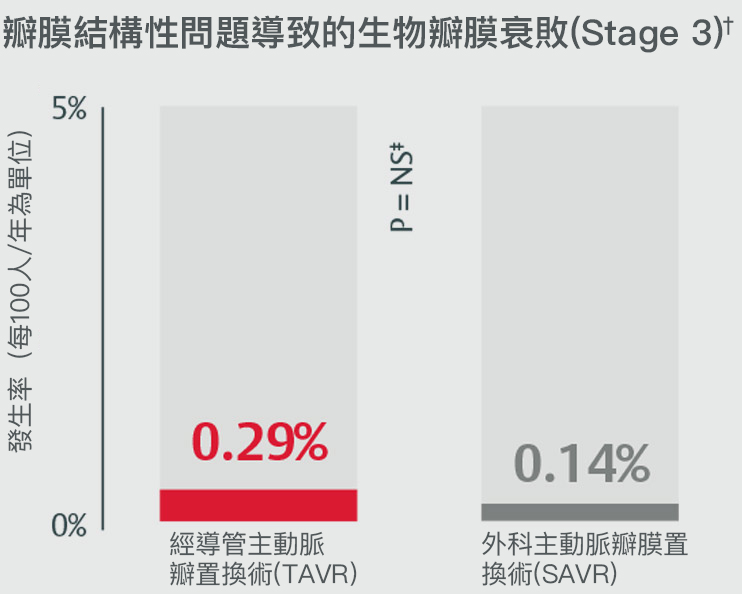
*Stage 2 & 3 (moderate & severe HVD): morphological valve deterioration AND +Δ mean gradient ≥ 10 mmHg with final mean gradient ≥ 20 mmHG§ and any of: -Δ AVA ≥ 0.3 cm2 (or ≥ 25%)§, -Δ DVI ≥ 0.1 (or ≥ 20%)§ OR ≥ 1 grade Δ transvalvular AR with final grade ≥ moderate.
†Stage 3 (severe HVD): morphological valve deterioration AND +Δ mean gradient ≥ 20 mmHG with final mean gradient ≥ 30 mmHg§ and any of: -Δ AVA ≥ 0.6 cm2 (or ≥ 50%)§, -Δ ≥ 0.2 (or ≥ 40%)§ OR ≥ 2 grade Δ transvalvular AR with severe final grade.
‡PARTNER II propensity matched data were analyzed for SVD rates, finding no statistically significant difference between SAPIEN 3 TAVR and SAVR for all endpoints except for all-cause BVF. Half of cases within all-cause BVF in SAVR were due to endocarditis (4 of 8); the majority of cases within SAPIEN 3 TAVR were due to paravalvular AR, a form of non-structural valve dysfunction (11/19).
§Compared to echocardiographic assessment performed 1 to 3 months post-procedure (or discharge if not available).
閱讀下一個主題SAPIEN 3瓣膜用在手術低風險的二尖瓣主動脈瓣患者身上也有傑出的表現9
PARTNER 3試驗:手術低風險之二尖瓣主動脈瓣患者的分析表格
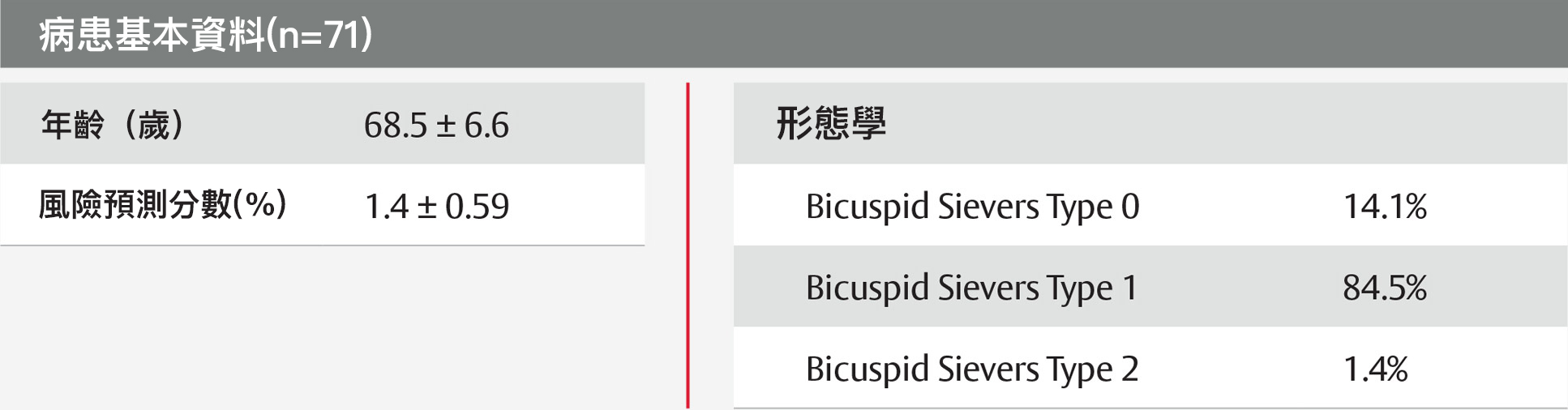
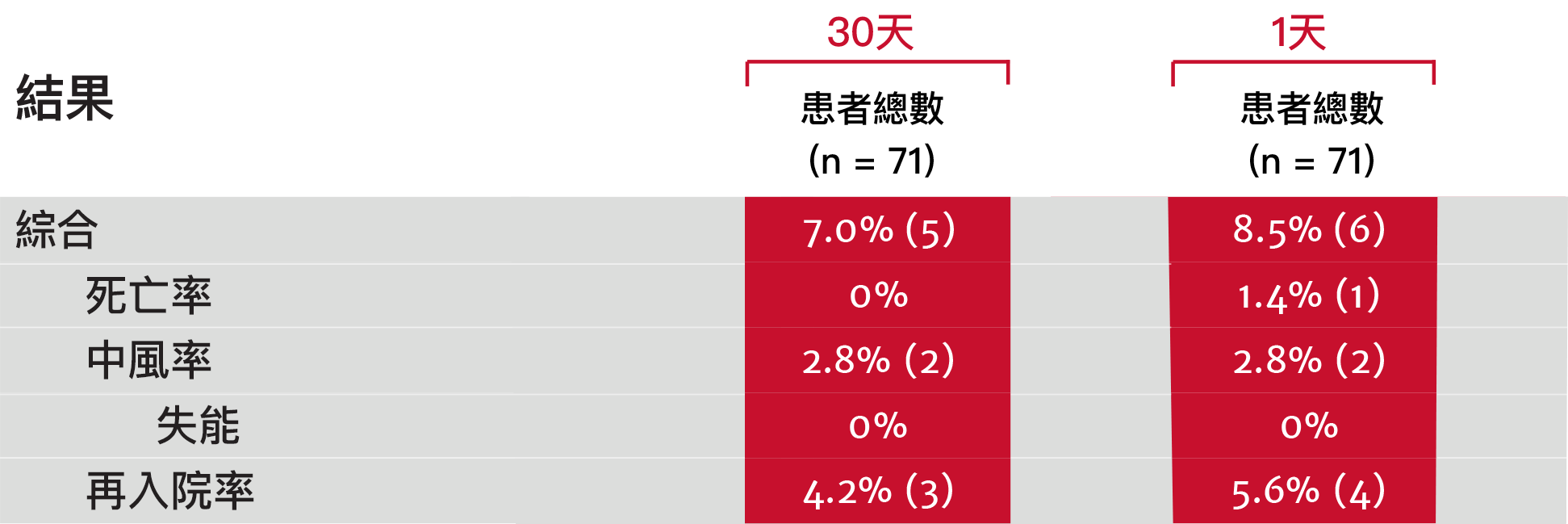
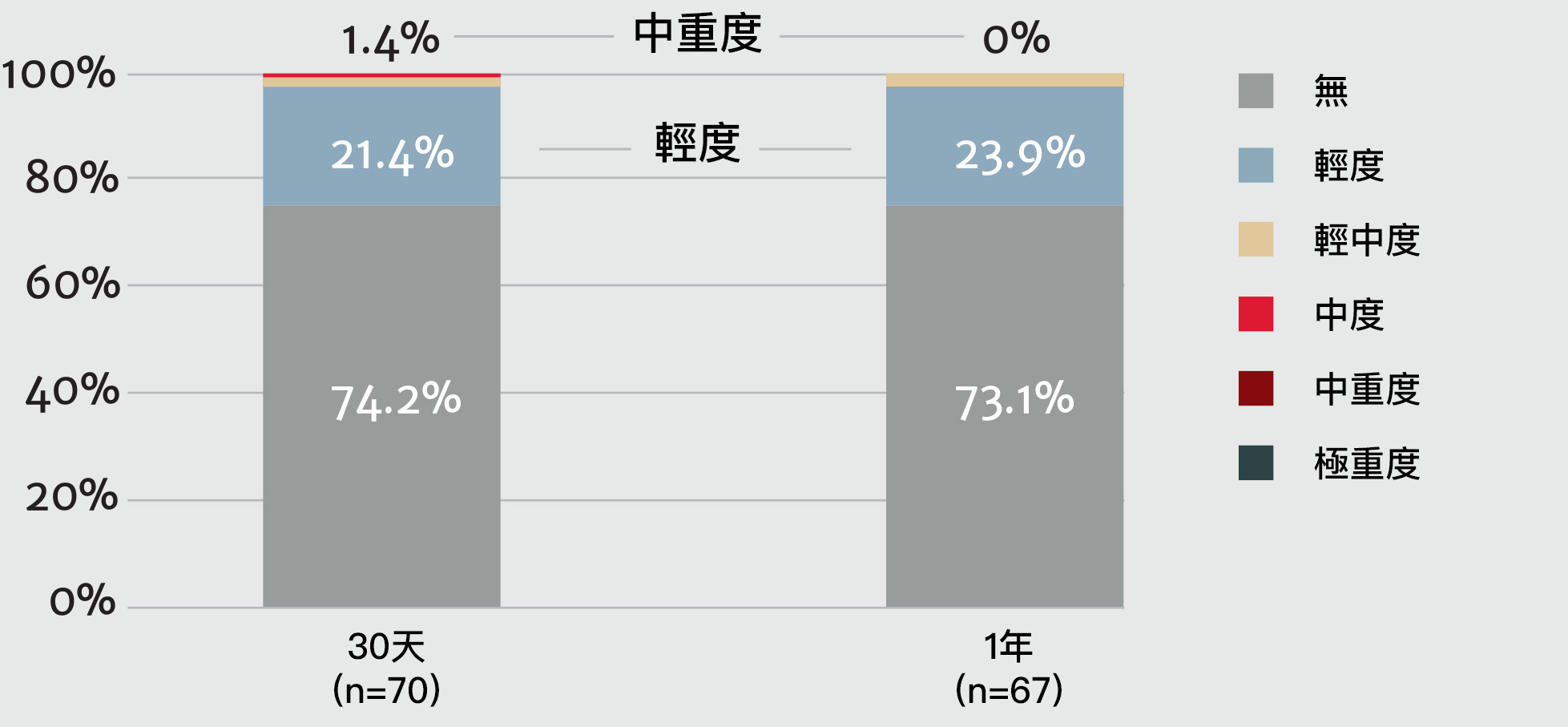
術後一年內無中重度瓣周漏(PVL)發生9
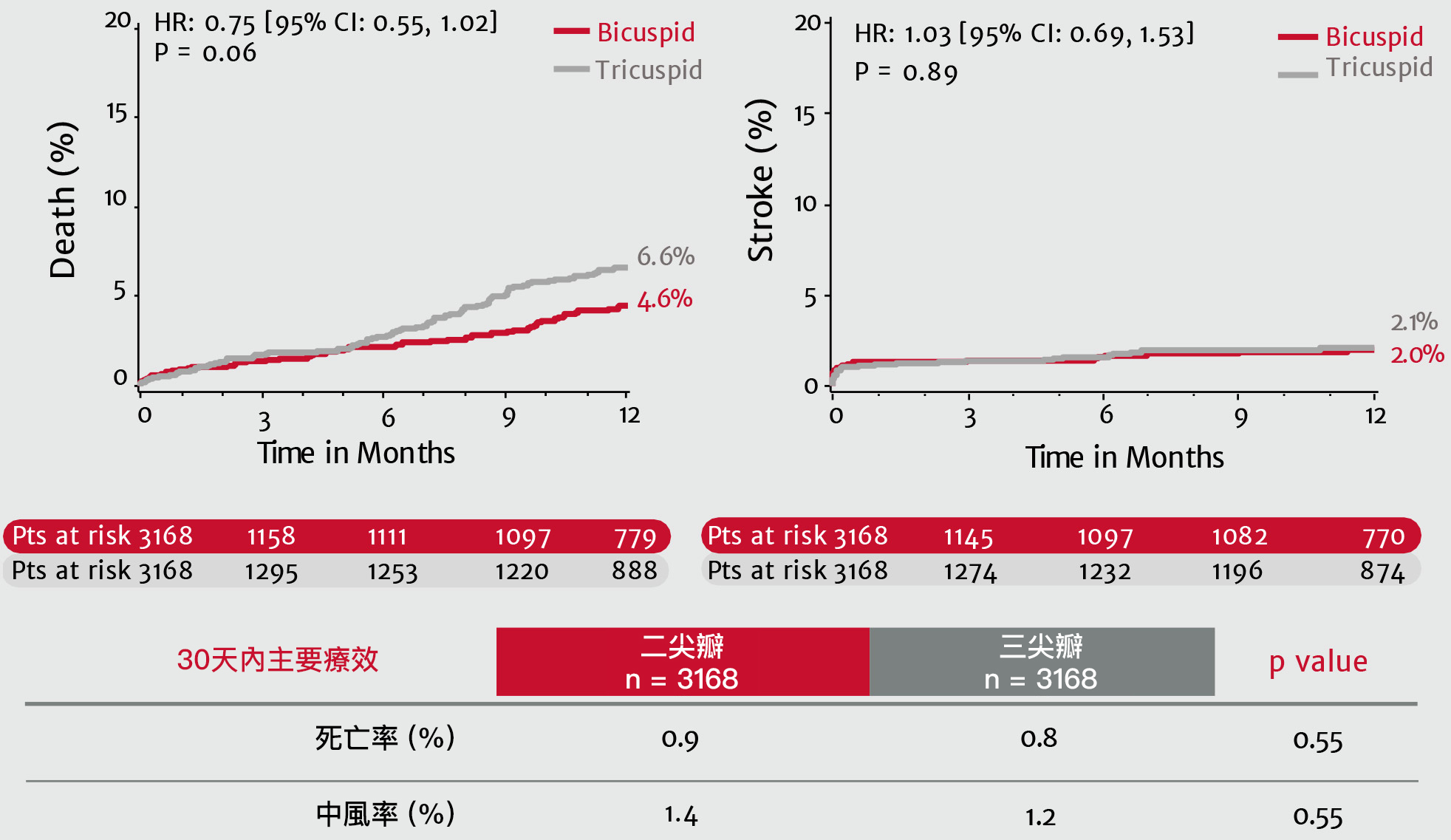
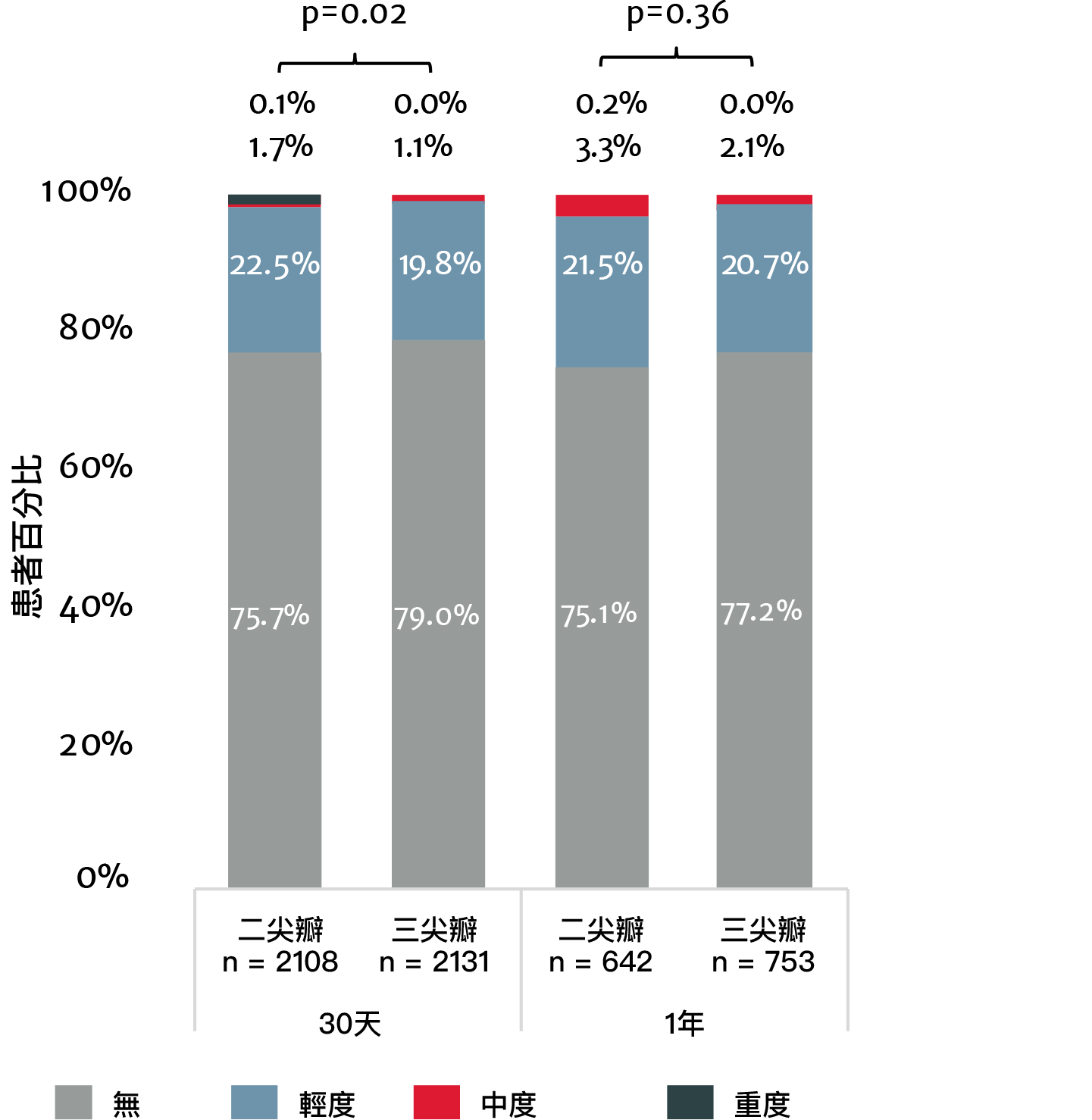
現實生活中,3100多名使用SAPIEN 3 瓣膜進行經導管主動脈瓣置換術的二尖瓣患者:一年內的死亡率及中風率與三尖瓣患者並無差異10
瓣周漏(PVL)
 閱讀下一個主題
閱讀下一個主題
The only valve approved for aortic THV-in-THV , mitral valve-in-valve (MViV) , and mitral valve-in-ring (MViR) *
Treatment options with SAPIEN 3 TAVR
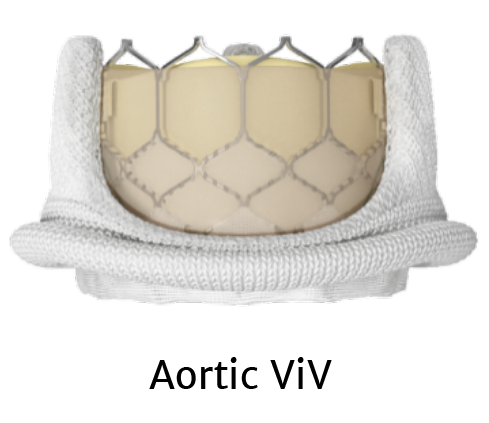

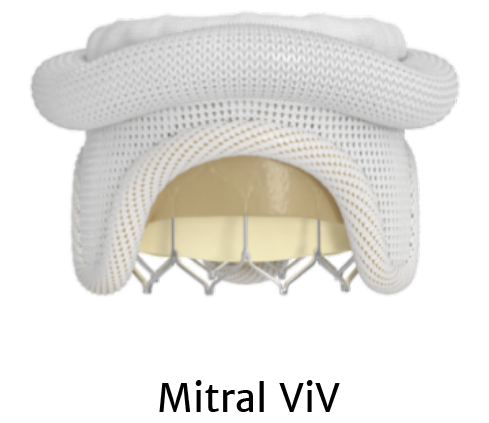

*For patients at high or greater surgical risk.
References: 1. Ochiai T, Chakravarty T, Yoon SH, et al. Coronary access after TAVR. JACC Cardiovasc Interv. 2020;13(6):693-705. 2. De Backer O, Landes U, Fuchs A, et al. Coronary access after TAVR-in-TAVR as evaluated by multidetector computed tomography. JACC Cardiovasc Interv. 2020; 13(21):2528-2538. 3. 2017 ACS Census Data, 2018 Medicare Quarterly SAF. 4. Pibarot, et al. Structural Deterioration of Transcatheter Versus Surgical Aortic Valve Bioprostheses in PARTNER II Trial. J Am Coll Cardiol. 2020. 5. Yudi MB, Sharma SK, Tang GHL, Kini A. Coronary angiography and percutaneous coronary intervention after transcatheter aortic valve replacement. J Am Coll Cardiol. 2018;71(12):1360-1378. 6. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expendable valve in low-risk patients. N Engl J Med. 2019;380(18):1695-1705. 7. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374(17):1609-1620. 8. Leon MB, Smith CR, Mack MJ, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597-1607. 9. Williams M.et al. The PARTNER 3 Bicuspid Registry for SAPIEN 3 TAVR in Low Surgical Risk Patients; Presented at TCT 2020; October 202, tctconnect.com. 10. Makkar RR, et al. Outcomes of Transcatheter Aortic Valve Replacement for Bicuspid Aortic Valve Stenosis in the Low-Surgical Risk Population. EuroPCR 2021 May. 11. Tarantini G, Fovino LN, Le Prince P, et al. Coronary access and percutaneous coronary intervention up to 3 years after transcatheter aortic valve implantation with a balloon-expandable valve. Circ Cardiovasc Interv. 2020;13:e008972. DOI: 10.1161/CIRCINTERVENTIONS.120.008972.