Providing an effective treatment.
See what the data says about how SAPIEN 3 TAVR/I can help your patients.
To you, it's about superior* outcomes for low-risk
symptomatic severe aortic stenosis (sSAS) patients.1
To him, it's about being there to watch
his grandson surf his first barrel.
To you, it's about superior* outcomes for low-risk
symptomatic severe aortic stenosis (sSAS) patients.1
To him, it's about being there to watch
his grandson surf his first barrel.
With nearly 15 years since its first commercial implant and over 763,000 patients implanted in over 75 countries around the world, the SAPIEN valve platform** provides an unparalleled experience.
*The PARTNER 3 trial provided SAPIEN 3 TAVR is superior to surgery on the primary endpoint of all-cause death, all stroke, and rehospitalization, and multiple pre-qualified secondary endpoints at one year.
**Includes SAPIEN, SAPIEN XT, SAPIEN 3, and SAPIEN 3 Ultra valves
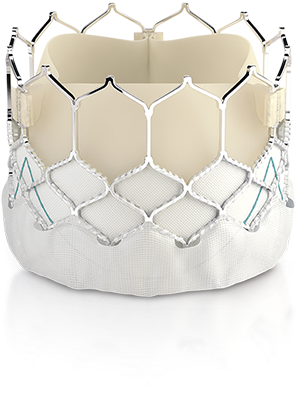
SAPIEN 3 TAVR/I demonstrated a 100% success rate of coronary access following TAVR/I implantation (68/68).2
The SAPIEN 3 valve has an intra-annular design, low frame height, and open cell geometry.

See what the data says about how SAPIEN 3 TAVR/I can help your patients.
In a study of real-world patients, with less than 0.2% of patients demonstrating moderate to severe PVL at discharge.3
The risk of death and disabling stroke is nearly 3 times lower with SAPIEN 3 TAVR/I than with surgery at one year for low-risk patients (1.0% for TAVR vs. 2.9% for surgery).1
SAPIEN 3 TAVR delivered permanent pacemaker 7.3% compared to 5.4% permanent pacemaker implantation for surgery at discharge for low-risk patients (p=0.21).1

The SAPIEN valve platform* allows for early discharge following treatment, so your patients can more rapidly get back to their normal lives.
*Includes SAPIEN XT, SAPIEN 3, and SAPIEN 3 Ultra
*Study includes both SAPIEN 3 and SAPIEN XT.
Thank you for signing up to the heartvalves.com newsletter. You will now receive emails on the latest developments and industry insights on heart valve innovation and technology.
References:
1. Mack MJ, Leon MB, Thourani VH. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med. 2019;380(18):1695-1705
2. Tarantini G, Fovino LN, Le Prince P, et al. Coronary access and percutaneous coronary intervention up to 3 years after transcatheter aortic valve implantation with a balloon-expandable valve. Circ Cardiovasc Interv. 2020;13(7):e008972.
3. Nazif T, Daniels D, McCabe J, Chehab B, et al. Real-world experience with the SAPIEN 3 Ultra TAVR: A propensity matched analysis from the United States. Presented virtually at TVT Connect 2020.
4. Wood DA, Lauck SB, Cairns JA, et al. The Vancouver 3M (Multidisciplinary, Multimodality, But Minimalist) Clinical Pathway Facilitates Safe Next-Day Discharge Home at Low-, Medium-, and High-volume Transfemoral Transcatheter Aortic Valve Replacement Centers: The 3M TAVR Study. J Am Coll Cardiol Intv. 2019;12(5):459-469.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
PP--EU-4686 v1.0